Journal of Astrobiology and Space Science Research, 6, 27-35, 2020
Mariko Kouduka1, Yuri Sueoka1, Yohey Suzuki1*
1Graduate School of Science, The University of Tokyo, 7-3-1 Hongo Bunkyo-ku,
Tokyo 113-0033, Japan.
Backward planetary protection involving the risk assessment of putative Martian life represents technological and social challenges for sample return missions from Mars. We aimed to develop procedures to inactivate microorganisms such as bacteria and viruses without losing the capability of life detection by high-sensitivity analytical measurements. In this study, near-saturated calcium chloride solution was tested for the short-term inactivation of model bacterial and viral organisms, which was followed by their entrapment in calcium carbonate. Cultivation-based assessments revealed that the near-saturated calcium chloride solution inactivated Escherichia coli and Bacteriophage T4 within one minute regardless of subsequent calcium carbonate precipitation. E. coli cells inactivated with the near-saturated calcium chloride solution and entrapped within calcium carbonate precipitates were embedded in hydrophilic resin, DNA-stained thin sections of which were observed by fluorescent microscopy. As a result, the E. coli cells were clearly visualized without cell ruptures. As the entrapment of microbial cells into CaCO3 grains has some advantages such as anti-scattering effect and minimizing the analytical inference for organic compounds, the method developed in this study has the potential to be applied to life detection procedures conducted by rovers and astronauts on Mars, as well as confinement facilities in space stations and on Earth.
Keywords: backward planetary protection, rock-hosted microorganisms, viral and bacterial disinfection, anti-scattering agent.
There is now considerable evidence that Mars at once time, had rivers, lakes, seas, and an ocean (Grotzinger 2014; Grotzinger et al., 2015; Yen et al., 2017) and may have been inhabited by bacteria (Joseph et al., 2019; Noffke 2015; Thomas-Keprta et al., 2009). What became of the surface waters of Mars is unknown. However, subsequent periods of freezing and warming, coupled with other geodynamic forces, could have preserved the remains of any organisms which may have populated Mars in the ancient past (Grotzinger 2014; Grotzinger et al., 2015; Wordsworth 2016). In addition to organics, some investigators have provided morphological evidence of what may be Martian organisms, i.e. fungi, algae and lichens (Joseph et al., 2020a, 2020b) though if they are biological or abiogenic is unknown. NASA's rover 2020 is specifically designed to search for evidence of past life, including chemical and biological fossils (Horgan et al., 2020; Onstott et al., 2019); and what may be fossils have also been detected on Mars (Baucon et al., 2020; Joseph et al., 2020b; Kaźmierczak, 2016). Likewise, the ESA-Roscosmos Rosalind Franklin rover is designed to hunt for biosignatures (the European Space Agency). The ultimate goal is to return these samples to Earth.
Sample return missions from Mars must have rigorous safeguards with respect to planetary protection, given that any forms of life present in Mars samples could be a potential source of extraterrestrial biological contamination on Earth. Hence, it is mandatory to conduct strict containment of any unsterilized samples and to conduct timely analyses of any unsterilized samples using the most sensitive analytical techniques. If any signs of the existence of a non-terrestrial replicating entity are found, the returned sample must remain contained unless treated by effective and neutralizing sterilization and related procedures. Containment facility guidelines for receiving samples from Mars and protocols for detecting and sterilizing extraterrestrial life and biohazards were drafted by NASA in 2002 (Rummel et al., 2002), followed by a documentation from a workshop for life detection from returned samples (Kminek et al., 2014). NASA has determined, however, that despite extensive efforts to sterilize equipment sent to Mars, up to 540 distinct colonies per square meter consisting of millions of spores and microorganisms, e.g. Bacillus, Staphylococcus, Micrococcus and Streptococcus spp. (La Duc et al., 2014; Puleo et al., 1977; Venkateswaran et al., 2012). Therefore, samples returned from Mars may include organisms that were sent to Mars; and therefore, we must be concerned with any mutational effects due to prolonged radiation exposure and horizontal gene transfer between organisms from Earth and those from Mars; and determine which may be native to Mars.
As sample containers from Mars need to be opened in containment facilities where the release of putative Martian organisms into the Earth’s biosphere should be prevented. To minimize the dangers from Martian samples, space stations could be alternative sample receiving facilities (Joseph et al., 2020a). However, there must also be ways to determine if sample returns contain Martian or Earthly organisms or both. Therefore, high-sensitivity analytical techniques are necessary to preserve the scientific integrity of sample returned to Earth, and to determine if the samples contain conclusive, definitive evidence of extraterrestrial life.
However, this may not be feasible due to the limitations from space, weight and electricity for instruments and labor in confinement facilities. For Martian samples to be allowed for high-sensitivity analytical techniques outside the confinement facilities (Rummel et al., 2002), sterilization and inactivation procedures should be strictly executed; which unfortunately, may deteriorate the sample quality for life detection.
Technological development is needed to minimize the risk of contamination by putative Martian organisms and to maintain the scientific value of returned samples. Previously, we developed a new method to visualize microbial cells in thin sections of rock fractures and clay fractions in drilled basalt lava after being embedded in hydrophilic resin, which is permeable with a DNA stain called SYBR-Green I (Sueoka et al., 2019). The hydrophilic resin has been demonstrated to be stable during high-sensitivity analytical techniques for life detection such as μ-Raman spectroscopy, high-resolution transmission electron microscopy (TEM) and nanoscale secondary ion mass spectrometry (NanoSIMS) (Suzuki et al., 2020).
Although the resin embedment is a promising procedure for life detection, the interference of the resin with subsequent organic analyses could be problematic. In addition, returned samples are desirably treated to reduce risks from the dispersal and growth of putative Martian organisms on Earth. To circumvent the analytical and safety issues, we aimed to develop new procedures for the inactivation of microorganisms, which is compatible with our resin-based life detection method. In this study, we tested the most characterized model organisms for bacterial and viral infections such as Escherichia coli and Bacteriophage T4 for the effects of procedures involved in CaCO3 precipitation to their ability for growth and infection.
2. Materials and Methods
Cultivation of E. coli and T4 infection to E. coli
E. coli (NBRC13168) and T4 (NBRC20004) were obtained from the NITE Biological Resource Center (NBRC), Japan. E. coli was cultured in Luria-Bertani (LB) medium (Wako Pure Chemical, Tokyo, Japan) at 30°C. T4 was infected to E. coli cultured in LB medium at an optical density (OD) of ~0.35. OD was measured at a wavelength of 600 nm using a spectrophotometer (DR2400; Hach Co., Loveland, USA). A conversion factor of OD600 1.0 = 5×108 cells/ml was used to calculate the cell number of E. coli after harvesting from the culture medium.
Inactivation experiments in the course of CaCO3 precipitation
Cultured cells of E. coli with and without T4 infection were harvested by centrifugation at 10,000×g for 10 min at 4°C. Cells were washed with 0.9% NaCl solution twice, and then suspended in 0.9% NaCl solution at a cell density of ~ 6.0×108 cells/ml. The cell suspensions with and without T4 infection were incubated in 5 M CaCl2 solution for one minute at RT. The CaC 2l -treated cells were inoculated in the culture medium. The inactivation of E. coli was evaluated by the lack of growth in the culture medium at RT for two days. The inactivation of T4 was evaluated by the lack of growth suppression of E. coli, when the CaCl2-treated cells were inoculated in the culture medium with E. coli cells grown at an OD of ~0.35. Some portions of the cell suspensions incubated with 5M CaCl2 solution were added with 5M NaHCO3 to induce CaCO3 precipitation. After 3 min, the CaCO3 -entrapped cells were inoculated in the culture medium with growing E. coli cells, as conducted for the CaCl2-treated cells.
Fluorescence microscopy detection of CaCO3-entrapped E. coli cells
To clarify whether microbial cells are detected after the inactivation experiments with CaCO3 precipitation, CaCO3-entrapped E. coli cells were embedded in LR White Resin (London Resin Co. Ltd., Aldermaston, England) as described by Sueoka et al., (2019). Briefly, CaCO3-entrapped E. coli cells were dehydrated twice in 100% ethanol for 5 min. After serial infiltration with 50%, 70%, 80%, 90% and 100% LR White in ethanol, resin-infiltrated CaCO3-entrapped E. coli cells were solidified with an accelerator reagent (London Resin Co. Ltd., Aldermaston, England) at RT for 5 min. After the tip of the solidified resin containing CaCO3-entrapped E. coli cells was trimmed and subjected to making 3-μm thick thin sections by an ultramicrotome (Reichert Ultracut
S; Leica, Wetzlar, Germany). The thin sec-
tions were stained with SYBR Green Ⅰ (TaKaRa Bio inc., Shiga, Japan) and observed using an epifluorescence microscope (BX51; Olympus, Inc., Tokyo, Japan) equipped with a charge-coupled device (CCD) camera (DP71; Olympus).
3. Results
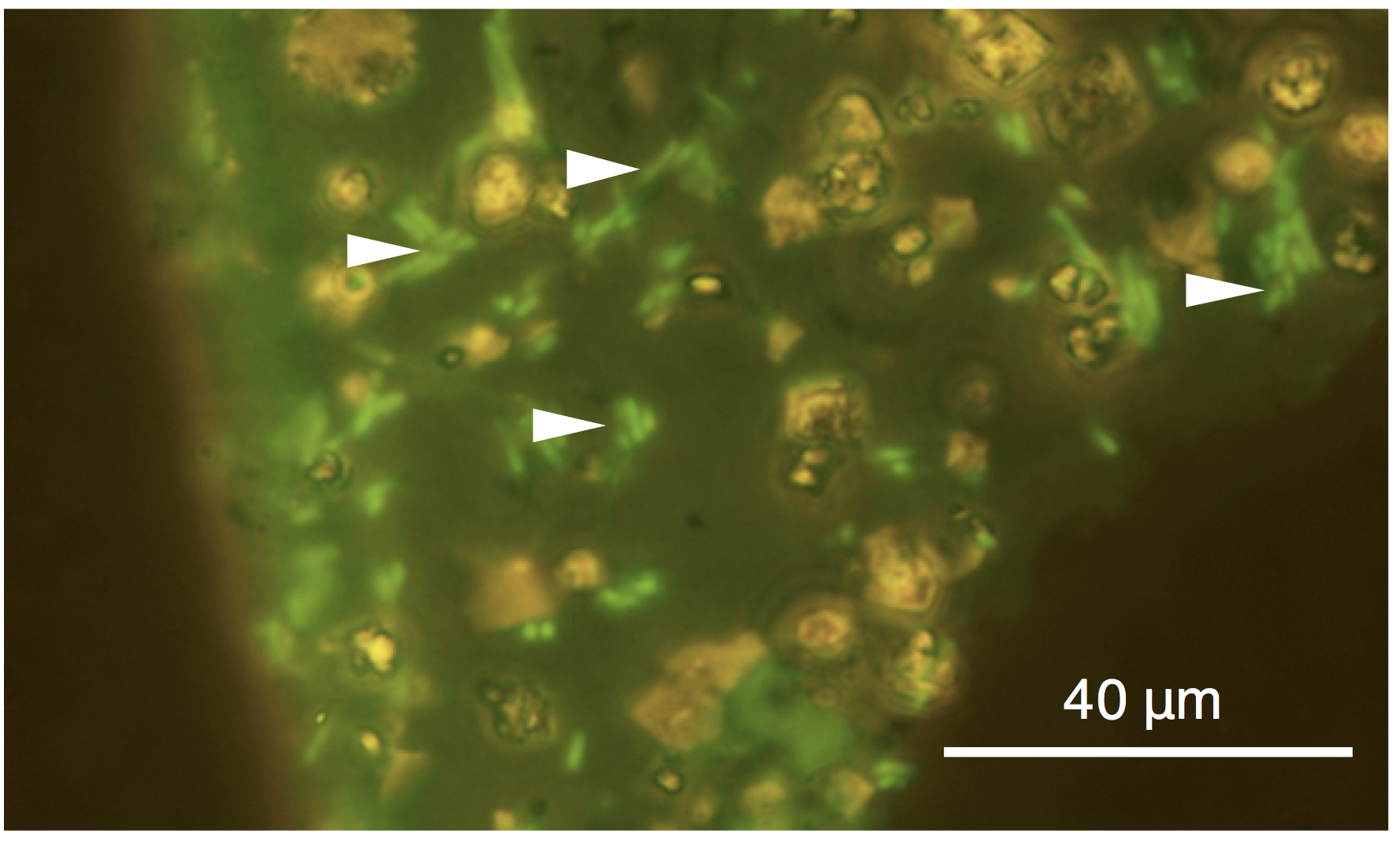
In the cell suspensions containing E. coli with and without T4 infection, stepwise additions of 5M CaCl2 solution and then 5M NaHCO3 resulted in CaCO3 precipitation. No growth of E. coli was observed in the culture medium, which demonstrated the inactivation of E. coli after 1-min incubation in 5M CaCl2 solution. 5M NaHCO3 addition after 1-min incubation in 5M CaCl2 solution also resulted in no growth of E. coli. As shown in Figure 1, the inactivation of T4 after 1-min incubation in 5M CaCl2 solution was demonstrated by the lack of growth suppression of E. coli, in contrast to T4-infected E. coli without CaCl2 treatment. The addition of 5M NaHCO3 after 1-min incubation in 5M CaCl2 solution also resulted in the lack of growth suppression of E. coli. CO3 precipitation was indicated by the formation of white solid material in the cell suspensions immediately after the addition with 5M NaHCO3. The white solid material was collected and embedded in the resin to make thin sections. In CaCO3 grains in a size range of ~100 μm, individual cells of E. coli stained with SYBR Green I were clearly visualized by fluorescence microscopy (Fig.2).
Figure 1. Evaluation of the loss of Escherichia coli infection by Bacteriophage T4 with and without CaCl2 treatment and CaCO3 precipitation. The growth of E. coli was measured based on optical density (OD600). E. coli was infected with T4 when the OD600 was 0.35.
Figure 2. Fluorescence microscopy image of SYBR Green I-stained Escherichia coli cells after CaCl2 treatment and CaCO3 precipitation. Arrows indicate the presence of E. coli cells in a CaCO3 grain.
4. Discussion
Rock fractures and clay fractions are expected to harbor putative Martian organisms. However, return samples with these characteristics are subjected to strict sterilization due to the high contamination risk. In this study, it is clarified that CaCl2 treatment followed by CaCO3 precipitation leads to the inactivation of E. coli cells, which remain to be detected by the method successfully applied to rock fractures and clay fractions in drilled basalt lava (Sueoka et al., 2019). These sample treatment procedures appear to be compatible with scientific studies in which organic compounds are characterized by spectroscopy and mass-spectrometry techniques such as Fourier-transform infrared spectroscopies, scanning transmission X-ray microscopy (STXM) and time-of-flight
secondary ion mass spectrometry (ToF-SIMS). These sample treatment procedures might be feasible enough for rovers and astronauts to conduct on Mars and containment facilities in space stations. Furthermore, physical trapping of microbial cells in large CaCO3 grains could help to reduce the dispersal risk of putative Martian organisms after sample return to Earth.
CaCl2 is added to nonalcoholic beverages, baked goods and dairy products at ~0.2% (U.S. Food and Drug Administration 2019) and in culture media for microorganisms (Balch et al., 1979). In this study, it was demonstrated that a near-saturated level of CaCl2 (5M) is instantly lethal to E. coli and T4. Mechanisms of Ca toxicity is likely explained by those known for heavy metals such as binding of Ca2+ to negatively charged functional groups such as carboxylate and phosphate in lipids, proteins and nucleic acids (Suzuki and Banfield 1999 and references therein). However, it should be noted that after CaCl2 treatment and CaCO3 precipitation, the cellular integrity was maintained for visualization with DNA staining. E. coli is a Gram-negative bacterium, whereas T4 is a non-enveloped, double-stranded DNA virus (Madigan et al., 2000). To make use of these treatment procedures for sterilization of putative Martian organisms, further tests are necessary for other types of cellular and non-cellular organisms and their dormant states such as spores and cysts.
5. Conclusions
In this study, it was aimed to develop new inactivation procedures, which are compatible with our newly developed life detection method for rock fractures and clay fractions embedded in hydrophilic resin. CaCl2 treatment alone is effective to inactivate model microorganisms for infectious deceases within one minute. CaCO3 precipitation was found to help preventing the interference with organic compound analyses and the dispersal of putative Martian organisms into the Earth’s biosphere.
De Pater I., and Lissauer J. J. (2015) Planetary sciences. Cambridge University Press.
Grotzinger J. P. (2014) Habitability, Taphonomy, and the Search for Organic Carbon on Mars: Introduction to Special Issue. Science, 343: 386-389.
Grotzinger J. P., Crisp J. A., and Vasavada A. R. (2015) Curiosity's mission of exploration at Gale Crater, Mars. . Elements, 11: 19-26.
Horgan B. H., Anderson R. B., Dromart G., Amador E. S., and Rice M. S. (2020) The mineral diversity of Jezero crater: Evidence for possible lacustrine carbonates on Mars.Icarus, 339: 113526.
Joseph R., Graham L., Büdel B., Jung P., Kidron G., Latif K., Armstrong R., Mansour H., Ray J., and Ramos G. (2020a) Mars: Algae, Lichens, Fossils, Minerals, Microbial Mats, and Stromatolites in Gale Crater. J Astrobiol Space Sci Rev, 3: 40-111.
Joseph R., Armstrong R., Kidron G., Gibson C. H. and Schild R. (2020b) Life on Mars? Colonies of Mushroom-shaped specimens in Eagle Crater. J Astrobiol Space Sci Res, 5:88-126.
Joseph R. G., Dass R. S., Rizzo V., Cantasano N., and Bianciardi G. (2019) Evidence of life on Mars. J Astrobiol Space Sci Rev, 1: 40-81.
Kaźmierczak J. (2016) Ancient Martian biomorphs from the rim of Endeavour Crater: similarities with fossil Terrestrial microalgae. In: Paleontology. Stratigraphy. Astrobiology. In commemoration of 80th anniversary of A.Yu. Rozanov. edited by SV Rozhnovs, Borissiak Paleontological Institute RAS, Moscow, pp 229-242.
Kminek G., Con ley C., Allen C. C., Bartlett D. H., Beaty D. W., Benning L. G., Bhartia R., Boston P. J., Duchaine C., and Farmer J. D. (2014) Report of the workshop for life detection in samples from Mars. Life Sci Space Res, 2: 1-5.
La Duc M. T., Venkateswaran K., and Conley C. A. (2014) A genetic inventory of spacecraft and associated surfaces. Mary Ann Liebert, Inc. 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA.
Madigan M., Martinko J., and Parker J. (2000) Brock: Biology of Microorganisms. Prentice Hall, Upper Saddle River, NJ
Noffke N. (2015) Ancient sedimentary structures in the< 3.7 Ga Gillespie Lake Member, Mars, that resemble macroscopic morphology, spatial associations, and temporal succession in terrestrial microbialites. Astrobiology, 15: 169-192.
Onstott T., Ehlmann B., Sapers H., Coleman M., Ivarsson M., Marlow J., Neubeck A., and Niles P. (2019) Paleo-Rock-Hosted Life on Earth and the Search on Mars: A Review and Strategy for Exploration. Astrobiology, 19: 1230-1262.
Puleo J., Fields N., Bergstrom S., Oxborrow G., Stabekis P., and Koukol R. (1977) Microbiological profiles of the Viking spacecraft. Appl Environ Microbiol, 33: 379-384.
Rummel J. D., Race M. S., DeVinenzi D. L., Schad P. J., Stabekis P. D., Viso M., and Acevedo S. E. (2002) A draft test protocol for detecting possible biohazards in Martian samples returned to Earth.
Sueoka Y., Yamashita S., Kouduka M., and Suzuki Y. (2019) Deep microbial colonization in saponite-bearing fractures in aged basaltic crust: Implications for subsurface life on Mars. Front Microbiol, 10: 2793.
Suzuki Y., and Banfield J. F. (1999) Geomicrobiology of uranium. Rev Mineral Geochem, 38: 393-432.
Suzuki Y., Yamashita S., Kouduka M., Ao Y., Mukai H., Mitsunobu S., Kagi H., D’Hondt S., Inagaki F., and Morono Y. (2020) Deep microbial proliferation at the basalt interface in 33.5–104 million-year-old oceanic crust. Commun Biol, 3: 1-9.
Thomas-Keprta K. L., Clemett S. J., Mckay D. S., Gibson E. K., and Wentworth S. J. (2009) Origins of magnetite nanocrystals in Martian meteorite ALH84001. Geochim Cosmochim Acta, 73: 6631-6677.
US Food and Drug Administration. CFR - Code of Federal Regulations Title 21. 2019.
Venkateswaran K., La Duc M., and Vaishampayan P. (2012) Genetic Inventory Task: Final Report, JPL Publication 12-12. Pasadena, CA: Jet Propulsion Laboratory, California Institute of Technology: 1-117.
Wordsworth R. D. (2016) The climate of early Mars. Annu Rev Earth Pl Sci, 44: 381-408.
Yen A., Ming D., Vaniman D., Gellert R., Blake D., Morris R., Morrison S., Bristow T., Chipera S., and Edgett K. (2017) Multiple stages of aqueous alteration along fractures in mudstone and sandstone strata in Gale Crater, Mars. Earth Planet Sci Lett, 471: 186-198.
* Corresponding Author: Yohey Suzuki
Received: June 22, 2020
Revision Received: June 28, 2020
Second Revision Received: July 1, 2020
Accepted for Publication: July 7, 2020
Published: 8/4/2020
1. Introduction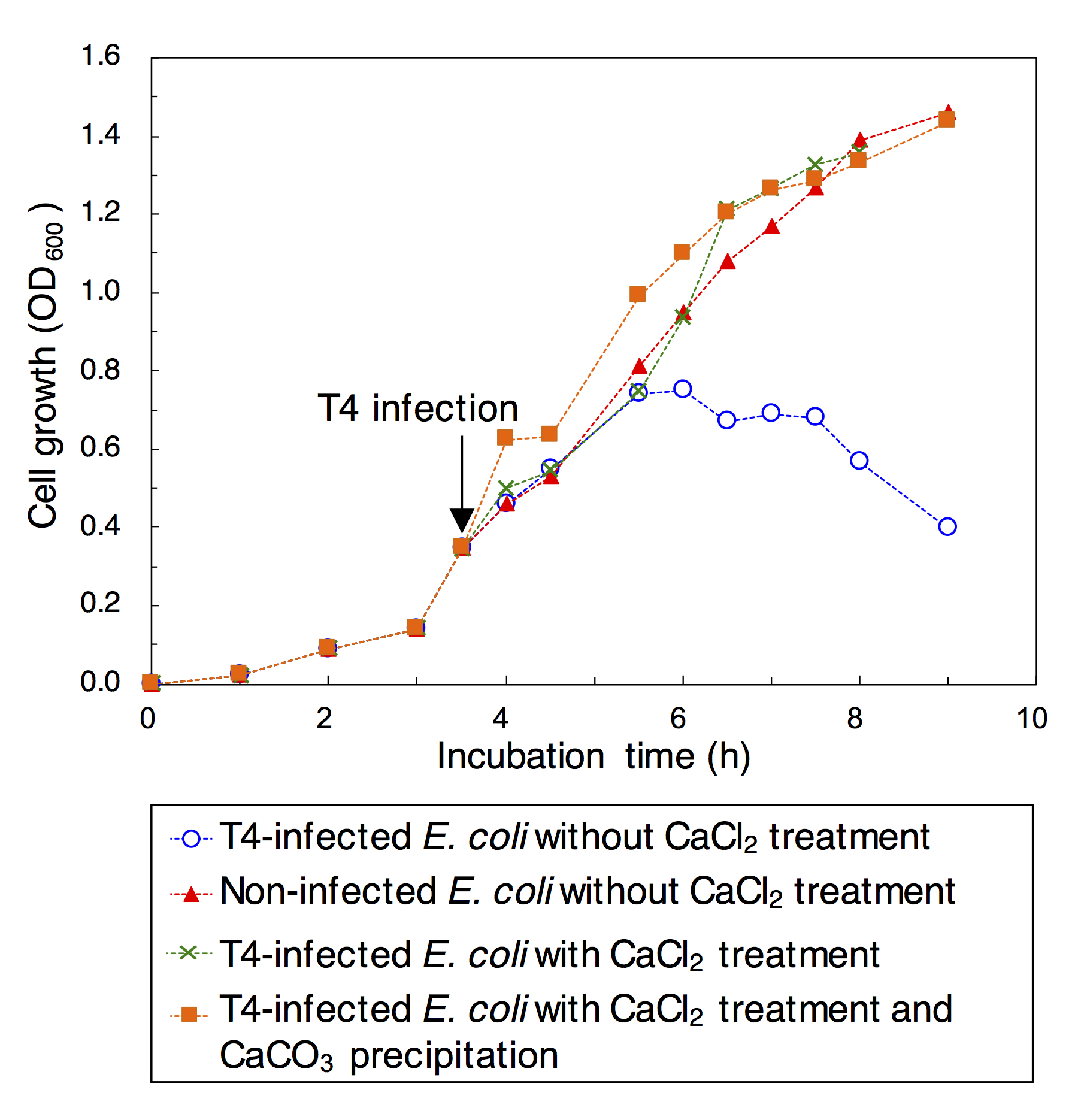
Acknowledgments: This work was supported, in part, by the Astrobiology Center Program of National Institutes of Natural Sciences (NINS) (AB021001 to Y.S.) and JSPS KAKENHI Grant Numbers 20H03319 (to Y. S.). We thank financial supporters to our work through a crowdfunding platform provided by Academist Inc (Tokyo, Japan): Takuya Hitomi, Shiro Toyoda, Katsuaki Watanabe, Shiro Yoshiaki, Yasuhiro Suzuki, Yoshiko Suzuki, Furuyama Taizo, Takeshi Tsuchiya and Yuki Ishibe. We also thank two anonymous reviewers for improving our manuscript.
Baucon A., Neto De Carvalho C., Felletti F., and Cabella R. (2020) Ichnofossils, Cracks or Crystals? A Test for Biogenicity of Stick-Like Structures from Vera Rubin Ridge, Mars. Geosci, 10: 39.


